A smarter use of organic nitrogen
Our society relies on the application of fertilizer to ensure sufficient crop yield for food production. The production of ammonia based fertilizers from inert nitrogen gas (N2) is responsible for about 1 to 2% of the worldwide energy consumption.
A large amount of this reactive nitrogen ends up in the wastewater we produce. Most notably the liquid fraction of animal manure and domestic wastewater, but also industrial waters from food processing plants may contain substantial amounts of ammonia. This needs to be either removed or contained to protect sensitive water bodies and to comply with current regulation.
Common practices remove the nitrogen from wastewater through an energy intensive process of nitrification and denitrification, converting reactive nitrogen back to N2.
The LIFE-NEWBIES project aims to shorten the nitrogen cycle by directly recovering nitrogen (in the from of ammonia, NH3) from wastewater using a combination of electrodialysis by bipolar membranes (EDBM) and gas stripping.
Earlier lab scale studies on this process to treat urine showed an effluent with a lowered TAN (total ammonia nitrogen) concentration and a product with potential use as a fertilizer (ammonium sulphate). Achieving a removal rate of 149 gN m-2 d-1 and recovery of 74% at a low energy input of 4.2 kWh kgN-1, the energy demand associated with nitrogen removal and production was substantially lower than using conventional methods*
How does it work?
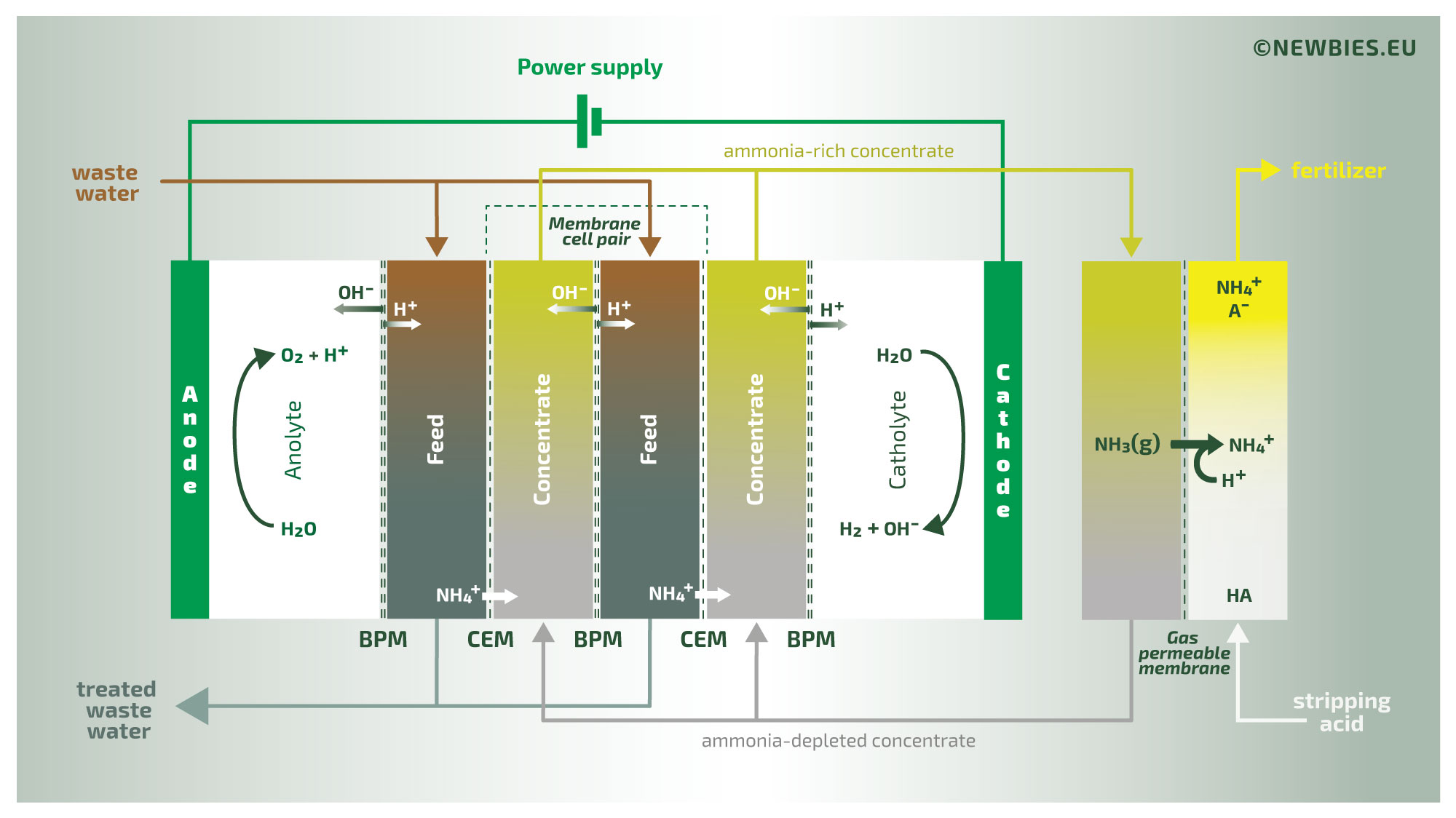
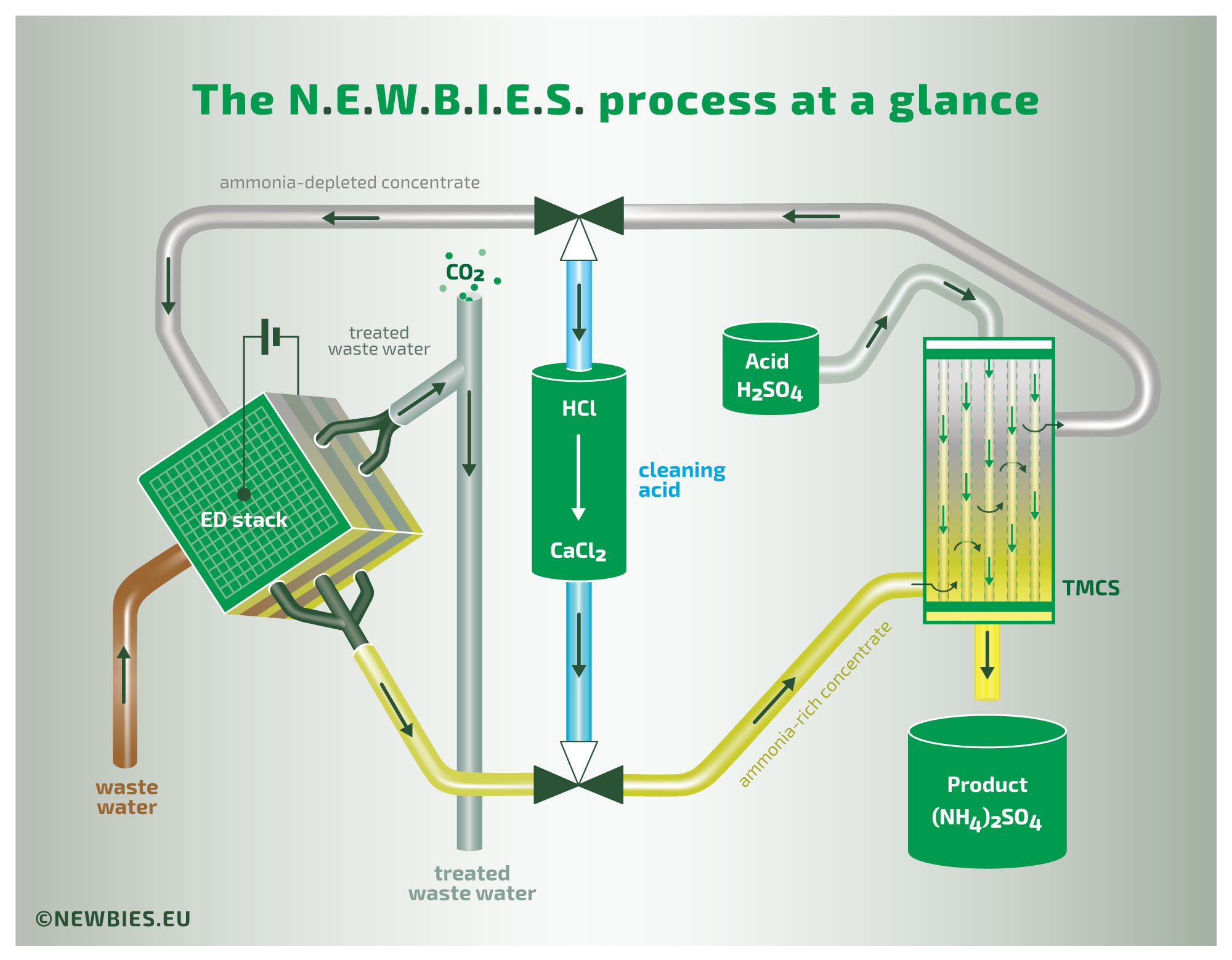
The illustration above provides an overview of the N.E.W.B.I.E.S. process. This process treats waste water containing relatively high concentrations of organic nitrogen in the form of ammonia. The waste water initially has a neutral or slightly acidic pH, with the nitrogen present as non-volatile ammonium. The waste water is introduced into an ElectroDialysis (ED) stack in which the ammonium is transported through ion-exchange membranes to a concentrate stream. The concentrate is then introduced into a gas membrane stripper (a process formally known as TransMembraneChemiSorption, or TMCS in short), where the gaseous ammonia diffuses across the membrane. Thus, by TMCS, the ammonia is transferred to a product liquid. The driving force of this last extraction is the addition of an external acid, in this example sulfuric acid, yielding a concentrated ammonium sulfate solution as the final product.
The second illustration provides a more in-depth view of the process. Clearly visible is the use of bipolar membranes as part of the ED-process. By using bipolar membranes the pH in this concentrate stream is very high. As a result of this high pH, the ammonium is converted into volatile ammonia. This volatilization of organic nitrogen is required for the subsequent TMCS to function properly.