Energy-Efficient Ammonia Recovery in an Up-Scaled Hydrogen Gas Recycling Electrochemical System
Authors: Philipp Kuntkea, Mariana Rodriguesa, Tom Sleutelsa, Michel Saakesa, Hubertus V. M. Hamelersa, and Cees J. N. Buismana,b
a Wetsus, European Centre of Excellence for Sustainable Water Technology, Oostergoweg 9, 8911MA Leeuwarden, The Netherlands
b Department of Environmental Technology, Wageningen University, Bornse Weilanden 9, P.O. Box 17, 6700 AA Wageningen, The Netherlands
Published in: ACS Sustainable Chem. Eng. 2018, 6, 6, 7638–7644 (8 May 2018)
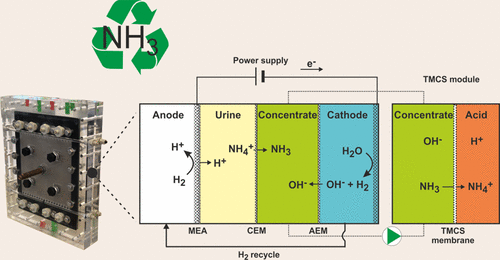
Abstract: Nutrient and energy recovery is becoming more important for a sustainable future. Recently, we developed a hydrogen gas recycling electrochemical system (HRES) which combines a cation exchange membrane (CEM) and a gas-permeable hydrophobic membrane for ammonia recovery. This allowed for energy-efficient ammonia recovery, since hydrogen gas produced at the cathode was oxidized at the anode. Here, we successfully up-scaled and optimized this HRES for ammonia recovery. The electrode surface area was increased to 0.04 m2 to treat up to 11.5 L/day (∼46 gN/day) of synthetic urine. The system was operated stably for 108 days at current densities of 20, 50, and 100 A/m2. Compared to our previous prototype, this new cell design reduced the anode overpotential and ionic losses, while the use of an additional membrane reduced the ion transport losses. Overall, this reduced the required energy input from 56.3 kJ/gN (15.6 kW h/kgN) at 50 A/m2 (prototype) to 23.4 kJ/gN (6.5 kW h/kgN) at 100 A/m2 (this work). At 100 A/m2, an average recovery of 58% and a TAN (total ammonia nitrogen) removal rate of 598 gN/(m2 day) were obtained across the CEM. The TAN recovery was limited by TAN transport from the feed to concentrate compartment.
Download: link to pdf
DOI: 10.1021/acssuschemeng.8b00457
License: CC-BY-NC-ND4.0
